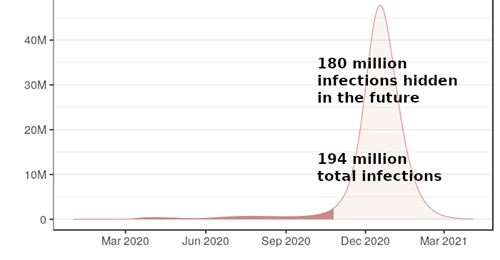
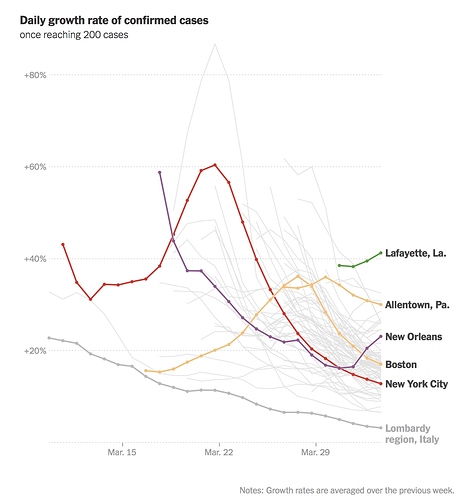
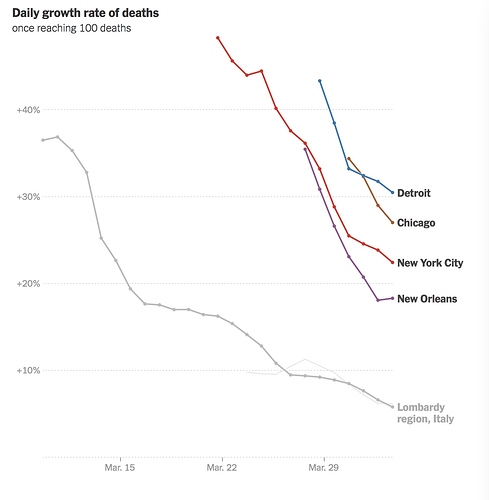
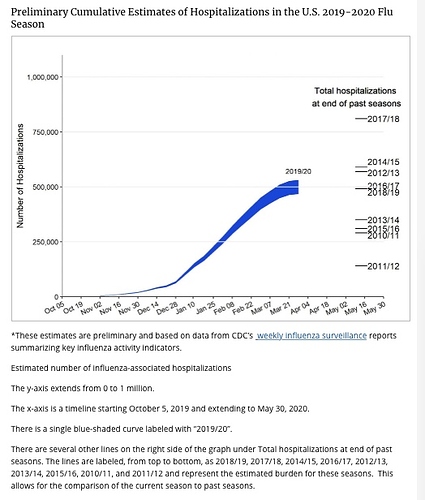
As was noted in an earlier post, confusing case-fatality rate with mortality is not a valid predictive model. Such early prediction based on limited data collection lacks widespread testing of the population. Added to the confusion is the so-called “death rate” that is properly a retrospective percentage of the population that was infected, and usually not available until some years after an outbreak is fully analyzed. As more and more data is collected, multiple problems requiring input with multiple variables will deliver a variety of more accurate solutions. The predictive models are changing daily with the increasingly more complexity, so stay tuned to reliable sources of information.
Note that Abbott’s rapid testing platform that is becoming widely available is an RT-PCR viral test. We were clearly behind on our available testing, but rapidly catching up. We have yet to have available the other half of the solution, a rapid antibody testing platform that is needed to separate the sick, from the recovered sick with antibodies that eliminate the virus, and those that have not yet caught the COVID-19 virus in the population. Such an antibody test is being developed through collaborative efforts around the world. Here is an excerpt from a very long article why the most common method of testing, RT-PCR tests, are not always reliable. Read the entire article if you would like to understand the full context:
COVID-19 testing
The RT-PCR tests are just one way to test for the virus – and it only detects it when people are still acutely infected, and the virus is still making all that RNA to make all the proteins it needs to make more of itself and infect more cells.
Once the virus is “conquered” by a person’s immune system, that viral RNA isn’t there anymore; however, evidence of the proteins made from it is – the immune response that allowed the body to fight off the virus involved making little proteins called antibodies that recognize specific pieces of the viral proteins as “foreign” and trigger an immune response.
After the initial infection, it takes a while for the body to develop antibodies against it – the process involves the viral proteins getting chopped up and their pieces placed “on display,” held by proteins jutting out from immune cells. Your body goes through a random “trial and error” approach to making antibodies that recognize (bind to) those viral protein pieces and then make more of the matching antibodies. More here: Antibodies – production, types & uses in the lab – The Bumbling Biochemist
Some of these antibodies stick around after the infection’s over to “keep watch” so that, if that same virus tries again, the immune system doesn’t have to go through the trial and error phase of finding an appropriate antibody. So, tests that look for antibodies can see if someone previously had the virus, even after they’ve recovered, and this can be used to trace cases back to see the line of transmission even if the transmitters are no longer symptomatic and don’t have the RNA that the RT-PCR tests could detect.
The antibody tests are quicker and they’re typically done on blood samples, but a downside with them is that, since they come from the immune response finally gaining some ground on the virus, they can’t detect the virus as early in an infection, while the RT-PCR way can.
There’s another fascinating story that I’ll post a link to for those who may be interested on how RT-PCR testing was even developed. Here is some excerpts from “the story” with the link included and a brief explanation of how it works:
Key ingredient in coronavirus tests comes from Yellowstone’s lakes
Today, those enzymes are a key component in polymerase chain reaction, or PCR, a method used widely in labs around the world to study small samples of genetic material by making millions of copies. This technique, which would have been impossible without the discovery of heat-resistant bacteria more than half a century ago, is now being used to boost the signal of viruses in most of the available tests for COVID-19.As the novel coronavirus sweeps around the world, testing has become the crux of tracking—and hopefully slowing—the pandemic’s advance. While authorities have been slow in making COVID-19 tests widely available in the U.S., the PCR process that is the vital backbone of the test is relatively simple and quick, thanks to a cluster of bacteria thriving in the thermal pools of Yellowstone.
Since the discovery of DNA’s elegant double helix in 1953, scientists have grappled with the challenge of studying these tiny genetic molecules. To see and understand different types of DNA, scientists needed large scale samples.
For years, PCR testing was “super laborious, it took forever,” says Julie Huber, an oceanographer at Woods Hole. “But now it’s so easy and routine.”
The COVID-19 test uses this same process—but with a few additional steps. The genetic material of the novel coronavirus is RNA, rather than DNA, which is similar but encodes its genetic instructions with different building blocks in just a single strand. The RNA of the virus is converted to DNA first. The test also includes a fluorescent tag that highlights the copies of the virus’ genetic material in a nasal swab. The more copies that are made with PCR, the brighter the sample.
Key ingredient in coronavirus tests comes from Yellowstone’s lakes
But what I really wanted to post is a major part of the “game changer” by Abbott labs. One should also note the system we had in place until very recently was tightly controlled centrally by the CDC, which is largely a data collection agency and policies regulated by the FDA. It is now decentralized into FDA certified commercial labs (private enterprise) and state health agencies, which are proving to be a much more efficient and logical solution for rapid large scale population testing:
ABBOTT LAUNCHES MOLECULAR POINT-OF-CARE TEST TO DETECT NOVEL CORONAVIRUS IN AS LITTLE AS FIVE MINUTES
The Abbott ID NOW™ COVID-19 test brings rapid testing to the front lines
Test to run on Abbott’s point-of-care ID NOW platform - a portable instrument that can be deployed where testing is needed most
ID NOW has the largest molecular point-of-care installed base in the U.S. and is available in a wide range of healthcare settings
Abbott will be making ID NOW COVID-19 tests available next week and expects to ramp up manufacturing to deliver 50,000 tests per day
This is the company’s second test to receive Emergency Use Authorization by the FDA for COVID-19 detection; combined, Abbott expects to produce about 5 million tests per month
Apologies again for a much too long post, but I have not had access to my home computer for some time! There is so much information available and things are going to scare the daylights out of much of the uniformed public, as well as those who are informed! Much of the “information” is just opinion and unreliable. I urge all of you to stay well informed, take precautions, and stay safe.
One more if you care to watch an investigative youtube from an Australian 60 Minutes feature, it is very scary!: